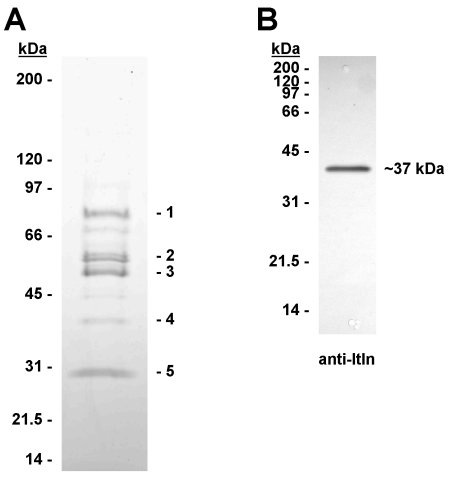
Fig. 1. SDS-PAGE identification of heparin agarose-binding human serum proteins. A: Heparin agarose was incubated with 10ml of human serum in the presence of 0.5 M NaCl (see Materials and Methods). The heparin agarose matrix was washed extensively, then eluted with SDS-containing buffer (100�l). Proteins in 10 �l of the eluate were separated by SDS-PAGE (4-20% gradient) and stained with Coomassie blue. The bands labelled 1 � 5 were excised and identified by tryptic peptide mass fingerprinting. B: Anti-intelectin western blot (12% SDS-PAGE) of proteins from serum (0.25 ml) eluted from heparin agarose with SDS-containing buffer. Itln1 was detected as a single band at approx 37kDa.
3. Results and discussion
3.1 Human serum Itln1 binds to heparin agarose
3.2 Binding of Itln1 to heparin agarose requires Ca2+ or Mg2+
3.3 Human Itln1 binds to unmodified agarose and Sepharose
Agarose is made up from linear repeating subunits of the disaccharide agarobiose (D-galactopyanosyl 3,6-anhydro-L-galactopyranose). Whilst Itln1 might recognise non-terminal D-galactopyranose in agarose, it is also possible that it has an affinity for 3,6-anhydro-L-galactopyranose, by virtue of the presence of the five-membered 3,6-anhydro ring. Agarose-binding proteins have been described before; for example, a Ca2+-dependent agarose binding protein was detected in chicken serum [15], and galectin-3 was also shown to interact weakly with the agarose matrix during Superose gel filtration [16]. This study shows that human Itln1 can also be regarded as an agarose-binding lectin. Knowledge of this property will be essential to avoid problems during attempted chromatographic purification of Itln1 on common agarose-based matrices. However, it should now be a simple matter to take advantage of this property in the design of highly specific purification protocols for native and recombinant Itln1.
3.4 Itln1 is preferentially eluted from agarose by pentoses [and ribonucleotides]
Acknowledgements
This work was funded by the Biotechnology and Biological Sciences Research Council (BBSRC award ref: BB/E009069). VIR was funded by The Carnegie Trust.
References
[1] S. Tsuji, J. Uehori, M. Matsumoto, et al., Human intelectin is a novel soluble lectin that recognizes galactofuranose in carbohydrate chains of bacterial cell wall. J. Biol. Chem. 276 (2001) 23456-63.
[2] S. Tsuji, M. Yamashita, A. Nishiyama, et al., Differential structure and activity between human and mouse intelectin-1: human intelectin-1 is a disulfide-linked trimer, whereas mouse homologue is a monomer. Glycobiology 17 (2007) 1045-51.
[3] A. Pemberton, P. Knight, J. Gamble, et al., Innate BALB/c enteric epithelial responses to Trichinella spiralis: inducible expression of a novel goblet cell lectin, intelectin-2, and its natural deletion in C57BL/10 mice. J. Immunol. 173 (2004) 1894-1901.
[4] A.T. French, P.A. Knight, W.D. Smith, et al., Expression of three intelectins in sheep and response to a Th2 environment. Vet. Res. 40 (2009) 53.
[5] J.K. Lee, J. Schnee, M. Pang, et al., Human homologs of the Xenopus oocyte cortical granule lectin XL35. Glycobiology 11 (2001) 65-73.
[6] R.Z. Yang, M.J. Lee, H. Hu, et al., Identification of omentin as a novel depot-specific adipokine in human adipose tissue: possible role in modulating insulin action. Am. J. Physiol. Endocrinol. Metab. 290 (2006) E1253-61.
[7] C.M. de Souza Batista, R.Z. Yang, M.J. Lee, et al., Omentin plasma levels and gene expression are decreased in obesity. Diabetes 56 (2007) 1655-61.
[8] Z. Wang, and T. Nakayama, Inflammation, a link between obesity and cardiovascular disease. Mediators Inflamm. 2010 (2010) 535918.
[9] J.C. Barrett, S. Hansoul, D.L. Nicolae, et al., Genome-wide association defines more than 30 distinct susceptibility loci for Crohn's disease. Nat. Genet. 40 (2008) 955-62.
[10] A.D. Pemberton, M.J. Rose-Zerilli, J.W. Holloway, et al., A single nucleotide polymorphism in intelectin-1 is associated with increased asthma risk. J. Allergy Clin. Immunol. 122 (2008) 1033-1034.
[11] J.K. Lee, L.G. Baum, K. Moremen, et al., The X-lectins: a new family with homology to the Xenopus laevis oocyte lectin XL-35. Glycoconj. J. 21 (2004) 443-50.
[12] S. Tsuji, M. Yamashita, D.R. Hoffman, et al., Capture of heat-killed Mycobacterium bovis bacillus Calmette-Guerin by intelectin-1 deposited on cell surfaces. Glycobiology 19 (2009) 518-26.
[13] Y.A. Suzuki, K. Shin, and B. Lonnerdal, Molecular cloning and functional expression of a human intestinal lactoferrin receptor. Biochemistry 40 (2001) 15771-9.
[14] A.T. French, J.A. Bethune, P.A. Knight, et al., The expression of intelectin in sheep goblet cells and upregulation by interleukin-4. Vet. Immunol. Immunopathol. 120 (2007) 41-6.
[15] S. Sugii, and Y. Hirota, Identification of a Ca(2+)-dependent agarose-binding protein in chicken serum. J. Vet. Med. Sci. 56 (1994) 143-5.
[16] S. Andre, H. Kaltner, T. Furuike, et al., Persubstituted cyclodextrin-based glycoclusters as inhibitors of protein-carbohydrate recognition using purified plant and mammalian lectins and wild-type and lectin-gene-transfected tumor cells as targets. Bioconjug. Chem. 15 (2004) 87-98.
[17] D.V. Semenov, T.G. Kanyshkova, V.N. Buneva, et al., Human milk lactoferrin binds ATP and dissociates into monomers. Biochem. Mol. Biol. Int. 47 (1999) 177-84.
(C) Alan Pemberton, 08/04/12
Human serum was incubated with heparin agarose in the presence of high salt (0.5M NaCl) in order to validate a method for pre-concentrating serum proteins with a high affinity for heparin. Five protein bands were identified (Figure 1A), of which band 4 was identified as Itln1 by peptide mass fingerprinting (10 peptides matched; 34% sequence coverage; MOWSE score 129; p<0.05). Bands 1, 2, 3 and 5 were also identified as IgM heavy chain, antithrombin-III, IgG2 heavy chain and Ig kappa chain, respectively (data not shown). Western blotting confirmed the presence of Itln1 as a single band at ~37kDa (Figure 1B). When the procedure was repeated with mouse, rat, dog, sheep, bovine, horse, rabbit, pig and donkey sera, all were negative for intelectin by western blotting apart from the donkey serum, which produced a faint signal at 37kDa (data not shown). This may be due to lack of expression of circulating intelectin(s) and/or a lack of affinity for heparin agarose and suggests caution in using these species as comparative experimental models for investigating the role of circulating intelectins.
When heparin agarose was incubated with human EDTA plasma, there was no evidence for the binding of Itln1 (Figure 2A(i)). However, where EDTA plasma was pre-treated with CaCl2 or MgCl2 to a final divalent cation concentration of 20mM, the Itln1 present in the plasma did bind to heparin agarose (Figure 2A(ii)). The lectin properties of Itln1 were previously shown by Tsuji et al. [1] to be Ca2+-dependent, therefore it seemed likely that Itln1 was binding to heparin agarose by the same mechanism.
Unexpectedly, there was no evidence for elution of heparin agarose-bound Itln1, when eluted with 200�l of TBS containing 0.5mg/ml heparin, heparan sulphate or chondroitin-6-sulphate (Figure 2A(iii)). This suggested that binding to heparin-agarose may be mediated by the agarose matrix itself. In order to test this possibility, the binding of serum Itln1 to heparin agarose was compared, in duplicate, to unmodified agarose (Figure 2B(i)). Both were equally effective, indicating that the agarose matrix was sufficient for binding to occur. The finding that Itln1 has high affinity for agarose warranted reinterpretation of the results of Tsuji et al. [1], who found that recombinant human Itln1 bound to D-galactose-coupled epoxy-activated Sepharose 6B. Since Sepharose is another form of crosslinked agarose, it was possible that Itln1 was likewise binding to this matrix at least partially via agarose chains. Therefore, we also used epoxy-activated Sepharose 6B, but instead of binding a sugar ligand, the matrix was immediately treated with ethanolamine, to quench the active epoxy group. Itln1 bound to Sepharose 6B, and was specifically eluted by EDTA (Figure 2B(ii)), thus indicating that the presence of a terminal D-galactose ligand was not essential. Notably, a small amount of Itln1 was eluted during washing of Sepharose 6B with TBS, which appeared to be due to the mechanical breakdown of this particular form of crosslinked agarose matrix during centrifugation.
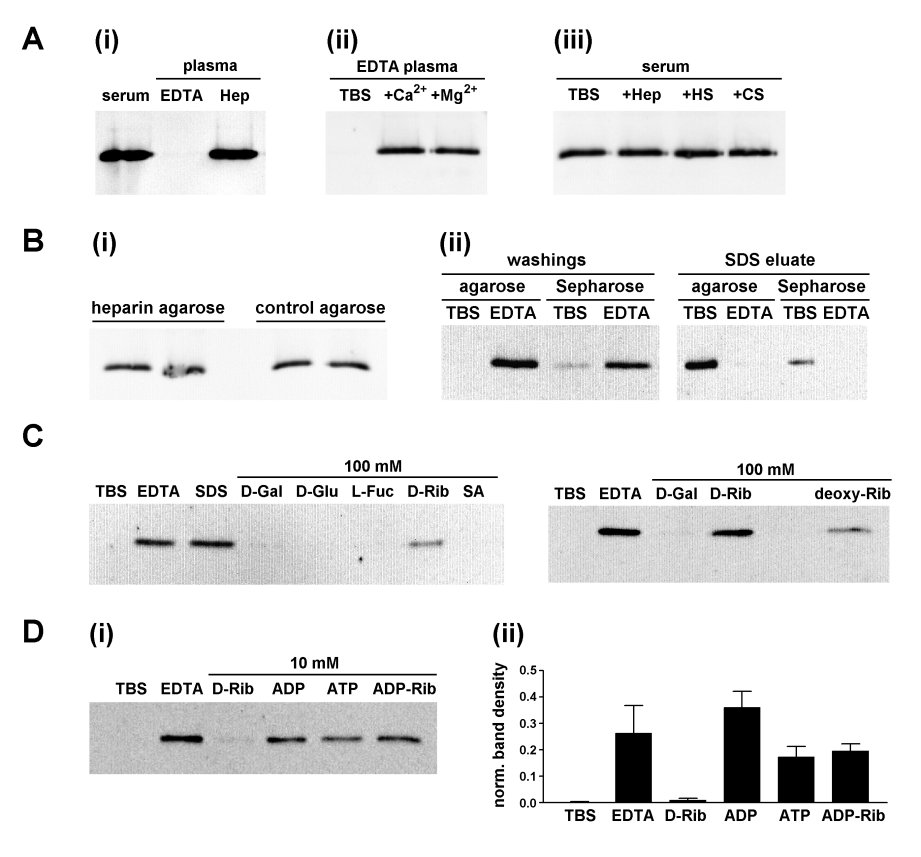
Fig. 2. Binding of Itln1 to heparin agarose and unmodified agarose. Anti-Itln western blots showing:
A (i) Comparison of the ability of heparin agarose to bind Itln1 from 250�l of human serum, EDTA plasma and heparin plasma. The presence of EDTA prevented binding of Itln1. (ii): The effect on Itln1 binding to heparin agarose of adding back Ca2+ (20mM CaCl2) and Mg2+ (20mM MgCl2) to EDTA plasma (250�l). (iii) The lack of effect of 0.5mg/ml heparin (Hep), heparan sulphate (HS) and chondroitin-6-sulphate (CS) on the binding of Itln1 to heparin agarose from serum (250�l).
B (i): The binding of Itln1 from human serum applied to heparin agarose and unmodified control agarose, performed in duplicate. The bound material was eluted with SDS and analysed by anti-Itln Western blotting, indicating that both matrices were equally effective in binding Itln1. (ii): Human serum was applied to control agarose and unmodified Sepharose 6B in duplicate spin columns. One column of each type was washed with TBS and another with TBS containing EDTA (10mM). Subsequently, the columns were eluted with SDS and samples analysed by anti-Itln Western blotting. Itln1 bound to both control agarose and Sepharose 6B. A proportion of Itln1 was lost during TBS washing, reflecting partial breakdown of the Sepharose 6B matrix during processing.
C: Human serum (125�l) bound to agarose (100�l) and eluted in TBS (100�l) containing variously EDTA (10mM), SDS (2% w/v), D-galactose (100mM), D-glucose (100mM), L-fucose (100mM), D-ribose (100mM), sialic acid (100mM) and 2-deoxy-D-ribose (100mM).
D (i): Comparison of elution of Itln1 from agarose by EDTA, D-ribose, and ribose-derivatives ATP, ADP and ADP-ribose (all at 10mM). This experiment was performed three times: a typical blot is shown, and the combined densitometry result (mean � SEM) for all three runs is shown in (ii). Integrated densities for individual bands are expressed relative to the total for all six lanes.
The carbohydrate-binding characteristics of Itln1 were previously reported by Tsuji et al. [1], using Itln1 bound to D-galactose-Sepharose-6B. A preference for elution by pentoses, such as 100mM D-ribose and 100mM D-galactofuranose, was demonstrated, compared to hexoses such as D-galactose and D-glucose [1]. Therefore, it was important to compare the elution characteristics of hexoses and pentoses using Itln1 bound to unmodified agarose. Here we found that Itln1 bound to agarose was eluted by monosaccharides (100mM) in a similar manner to that previously described, i.e. D-glucose and L-fucose were ineffective and D-galactose eluted a trace of Itln1, while the pentoses D-ribose and 2-deoxy-D-ribose were more effective (Figure 2C). We took the opportunity to compare the ability of D-ribose with other ribose-related compounds (i.e. the ribonucleotides ATP, ADP and ADP-ribose) in eluting Itln1 from agarose. It was found that, at 10mM concentration, D-ribose eluted Itln1 poorly compared to the ribonucleotides tested (Figure 2D). *** The following discussion is incorrect, since the ribonucleotides used are actually chelating agents and most likely eluted Intln-1 by virtue of this property, rather than competitive binding to the lectin binding pocket. *** [This shows that not only does the affinity of Itln1 for pentoses extend to ribose-containing ribonucleoties, but these also appear to be much more effective. This is the first demonstration of the ribonucleotide binding property of intelectins. It is intriguing that this should be the case, since lactoferrin, a known ligand of Itln1 [13], also binds ATP [17]. Therefore, ATP and/or other ribonucleotides may act as ligands or co-factors in the biochemical functions of Itln1, which at this moment remain obscure.]