Most of my career has been spent discovering and understanding the role of proteins that are expressed by the body in response to gastrointestinal parasites. As part of this work, I discovered that one of these proteins, intelectin, is produced by goblet cells, which are known for producing the glycoprotein mucin which is the main structural component of mucus. So in order to understand how intelectins function when expressed by goblet cells, it became more important for me to understand what components go to make up mucus and how the constituent proteins interact with each other.
Mucus is essential for protecting the GI tract from the environment, including damage by the massive load of commensal and potentially pathogenic bacterial contained within the digestive tract. The gastrointestinal mucus layer is a continuous layer covering the entire epithelial surface of GI tract, with the possible exception of some areas of lymphoid cells. Atuma et al. (2001) found that the mucus layer (in rats) was deepest in the stomach and colon and thinnest in the small intestine. Mucus is composed of two distinct main layers – an outer “loose” mucus layer that can be aspirated with a pipette, and an inner “firmly adherent” mucus layer that cannot be removed from the underlying epithelium by suction.
The epithelial layer itself is generated from stem cells that are located near the base of crypts. These divide and the new cells either migrate up the crypt, differentiating into epithelicytes, goblet cells or enteroendocrine cells, or migrate downwards, becoming Paneth cells, which line the base of the crypts (Barker et al., 2008)
The cells migrating up the intestinal crypts take approximately 4-5 days to do so, and are then extruded from the top. Thus almost the entire epithelial layer is renewed in a matter of days (Paneth cells have a longer lifespan). Indeed, it was estimated that the small intestine loses approximately 84g of protein per day (da Costa et al., 1971), of which approx 10% is due to loss of epithelial cells; the remainder coming from extracellular sources, such as plasma. This material must be resorbed further down the intestine. Normal exfoliated epithelial cells undergo a form of programmed cell death, rapidly degenerating and are not detected in stool (although there is some debate about this - see Bertrand, 2011).
My question is this: do exfoliated epithelial cells degenerate within the mucus layer and become incorporated into it?
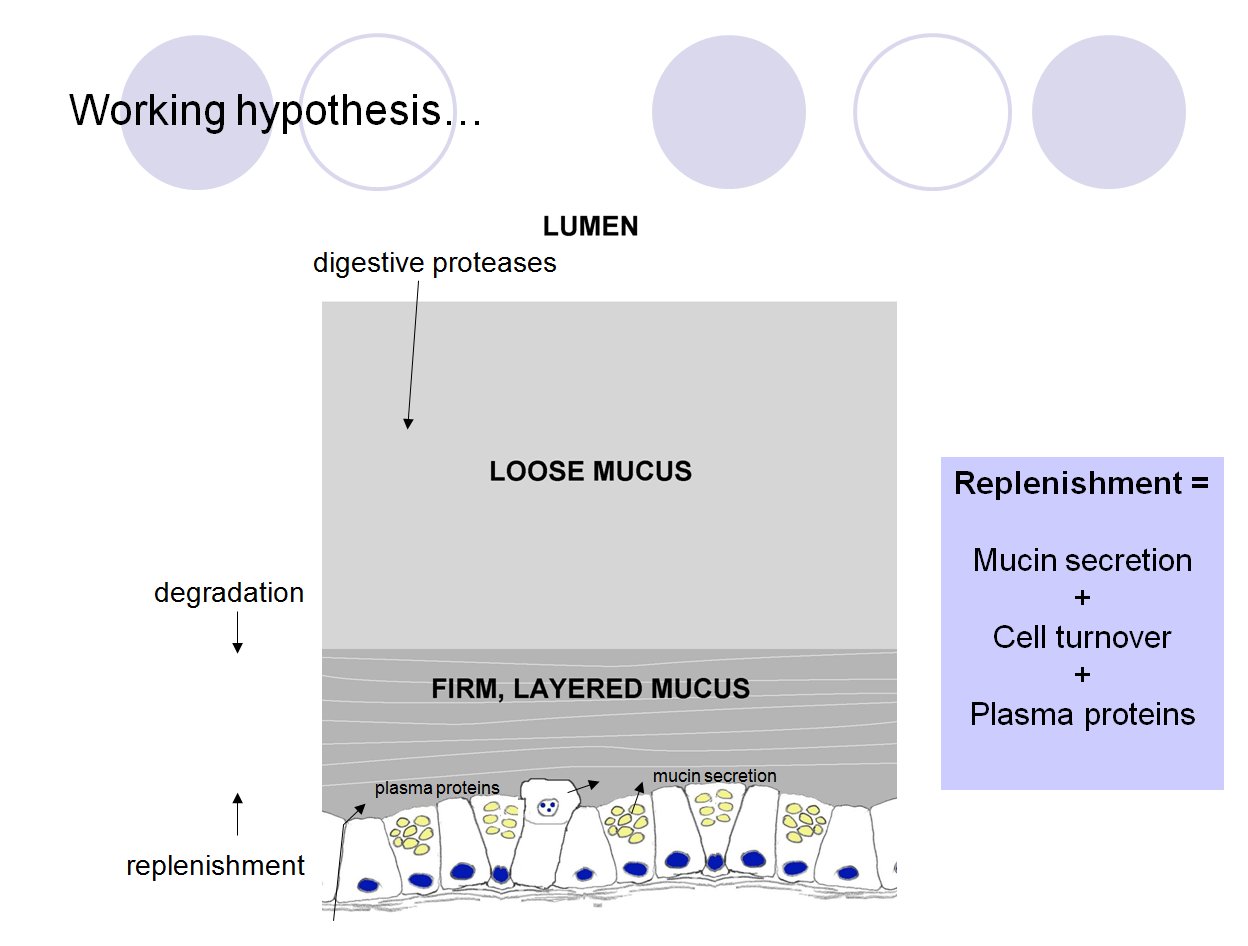
Schematic diagram summarising the hypothesis that GI mucus
is composed of the product of epithelial secretions,
exfoliated epithelial cells and transudated plasma. These
continually replenish the firmly adherent mucus layer, which
is continually attacked by the action of proteases and other
enzymes from the luminal side. Partially degraded firm mucus
becomes the loosely adherent layer. (Slide taken from my
presentation at WAAVP, Calgary, 2009)
It seems to make sense that if the firmly adherent mucus layer cannot be removed then it may form a sufficient barrier to exfoliated cells to prevent at least some of them from moving freely through the mucus and into the lumen for digestion. They would then degenerate in the presence of transudated plasma and mucins released from goblet cells; all of these components combining to form the fabric of the mucus layer. If this is the case, then the rate of cell turnover, mucin release and plasma transudation are all likely to affect the composition and structure of the resulting mucus. This model could be used to rationalise some of the changes in protein expression that occur within the epithelium during parasitic infections. It would also mean that exfoliated epithelial cells are not mere travellers through the mucus layer en route to destruction, but are actually essential for its formation and therefore could be one of the principal drivers of the rapid turnover of the GI epithelial layer.
This idea sparked a whole load of other questions about how the various components would interact, and how to show this experimentally. I made a start by trying to understand how the most abundant proteins from exfoliated cells, i.e. histones, once released from the confines to the nucleus, would interact with the other mucus components. The easiest of these to start with was plasma proteins. So a few experiments in adding histones to plasma, coupled with some background reading, opened up whole new areas to explore. I will confine myself here to two observations: 1) histones are lethal to small intestinal epithelial cells, but only in the absence of plasma proteins - i.e. plasma proteins protect the epithelial surface from damage; and 2) histones act as non-covalent cross-linkers, aggregating a certain subset of plasma proteins, including fibrinogen and lipoproteins. The crosslinking property of histones may be important in generating mucus structure. Some of this work was published in Proteomics (Pemberton at al., 2010) but it needs following up with studies of mucus itself.
It should be said that as someone who is not an expert in
mucus, I freely acknowledge my approach here to be quite
simplistic. This stuff might still give some food for thought,
but for real progress in understanding mucus, you should keep
an eye on these guys:
Jeff Pearson's lab in Newcastle http://www.ncl.ac.uk/camb/staff/profile/jeffrey.pearson
Gunnar Hansson's group in Gothenburg http://www.medkem.gu.se/mucinbiology/.
Dave Thornton's lab in Manchester: http://www.ls.manchester.ac.uk/research/researchgroups/cellmatrixresearch/people/?personid=1610
(NB: other mucus experts are available!)
References and further reading
Barker N, van de Wetering M, Clevers H. The intestinal stem cell. Genes Dev. 2008;22:1856-1864.
Pemberton AD, Brown JK, Inglis NF. Proteomic identification of interactions between histones and plasma proteins: implications for cytoprotection. Proteomics. 2010;10:1484-93. [NB:- this paper is not "open access". If you don't have institutional access to Proteomics, you may download an unformatted author version here: (1.16 MB)]